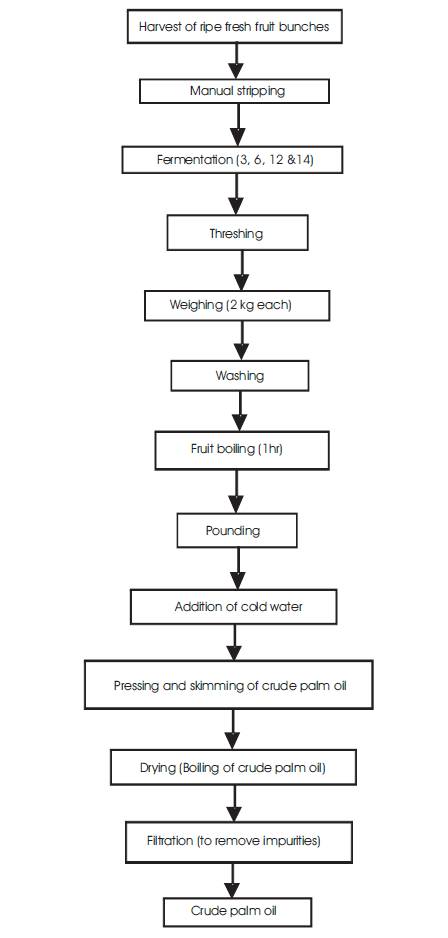
Figure 1. The flowchart of the Traditional Processing Method involved in the Process
This study evaluated the effect of fermentation time on the physicochemical characteristics of palm oil. The palm fruit samples used were divided into two groups with each being divided into four portions. One group was boiled during processing while the other group was not boiled. The two groups were evaluated and processed in batches separately, for three days, six days, twelve days, and fourteen days of fermentation using a traditional method of processing. A comprehensive range of physicochemical properties, namely: free fatty acid, moisture content, peroxide value, saponification value, iodine value, refractive index, freezing point, flash point, smoke point, and specific gravity were determined. The coefficient correlation of the effect of fermentation showed that, there were significant differences (P < 0.05) in the FFA, Iodine Value, Moisture Content, Saponification Value, Smoke point and Freezing Point, but no significant difference (P > 0.05) in Peroxide Value, Flash Point and unboiled samples for specific gravity and refractive index. Three days and six days of fermentation were seen to be ideal for the processing of palm oil as the values obtained gave the best product characteristics that are acceptable for international quality standards for palm oil. There is a need to avoid storing palm fruits beyond 6 days of fermentation before processing in order to produce high quality palm oil
Palm oil (Elaeis guineensis), a yellowish-red or orange-red oil extracted from the mesocarp of fruits of the oil palm tree is processed using various methods, and these techniques differ in the level of mechanisation, and interconnecting material transfer mechanisms. The scale of operations also differs at the level of processing and this affects the quality of the final product (Poku, 2002) .
The acceptable state of palm oil in the international market is largely dependent on the physiochemical properties of the oil which include: Free Fatty Acid (FFA), Iodine Value (IV), Peroxide Value (PV) moisture, impurities content, colour, taste, aroma, melting point, tocophenol, and tocotrienol contents (Cornelius, 1977; Johansson and Pehlergard, 1977; Hartley, 1988; Purseglove, 1995; Edem, 2002).
Fermentation is one of the few methods of preservation which actually enhances the nutritional value of a food. Fermentation is a natural method of concentrating raw food materials, and preserving them. Fermentation process retains and amplifies the positive health effects of organic virgin red palm oil and process the red palm oil through a proprietary non-heating natural enzymatic fermentation. The process can take up to three weeks and is carefully handled throughout the process to ensure the oil is clean and natural. However, excessive fermentation could lead to unpleasant odour and rancid product (Herbalzym, 2012).
Ohimain, et al. (2012) assessed the quality of crude palm oil produced by smallholder processors in Rivers state, Nigeria. Their study investigated the chemical quality of Crude Palm Oil (CPO) processed by smallholder processors at Elele, Rivers State, Nigeria. The mean result obtained showed that, the Free Fatty Acid (FFA) of the palm oil sample ranged from 8.44 – 10.30%, moisture content 13.70 – 18.21%, the impurity level 5.48 – 12.52%, and the peroxide value ranged from 1.20 – 1.90 MeqO2 kg-1 .The Analysis of variance showed that, there were significant differences (P < 0.05) in the FFA, impurity level, and moisture content, but no significant difference (P > 0.05) in peroxide value. The high values of FFA, impurity and moisture beyond recommended limits, which is caused by the method of processing, could limit the use of the oil beyond domestic consumption. They recommend the upgrading of the traditional processing method used by smallholder processors to modern and efficient mechanical methods.
Mbata and Orji (2008) studied the effect of extraction methods on the quality and spoilage of Nigerian palm oil. They evaluated the effect on properties and storability of the extraction oil. Three principal extraction methods were identified and included mainly traditional small scale, semi-mechanized and mechanized methods. Traditional methods were found to be classifiable into extraction by fermentation, wet extraction and extraction by frying. The oil extracted through the mechanized method had lower Free Fatty Acid (FFA), moisture and impurities than that extracted by the other methods. Oil extracted by the traditional methods, especially those produced by the fermentation method had the poorest quality. There were no differences in taste and aroma of fresh palm oil extracted through the different methods, but sharp differences exist among them in taste and aroma after storage for eight months. All methods produced low quality palm oil in terms of FFA, moisture and impurities when compared with international standards for export quality palm oil.
The effect of processing equipment and duration of storage of palm fruits on palm oil yield and quality in the Kwaebibrem District, Ghana was studied by Adjei-Nsiah, et al. (2012). The major processing practice identified to have a major effect on quality of processed palm oil wasduration of storage of palm fruits before processing. Three categories of processing equipments were identified; digester screw press, hand-operated screw press, and digester with separate hand-operated hydraulic press. These equipments were used to process palm fruits stored for 6 and 15 days after harvest to determine their effect on oil extraction rate and oil quality. The digester screw press had the highest extraction rate and oil yield of 13.5 and 25.5% respectively, while the digester with separate handoperated screw press had the lowest extraction rate and oil yield of 11.7 and 22.1% respectively. The two durations of storage of palm fruits before processing however did not have any significant effect on the extraction rate. However, both the processing equipment and storage durations had significant (P<0.05) effect on the resultant quality of crude palm oil processed. The study suggests the need to avoid storing palm fruits before processing in order to produce high quality crude palm oil.
The objectives of this study include:
The palm fruits used in this research work were purchased at Nwafia-Umuoguduakpu Market in Ngbo Ohaukwu Local Government Area of Ebonyi State.
Production of palm oil from palm fruit was done by employing traditional method of production. Figure 1 shows the unit operation to be involved in the process. In this process, bunches were harvested and the size was reduced immediately. The reduced size palm fruits were divided and hipped into four different portions where they were covered properly for various days of fermentation.

Figure 1. The flowchart of the Traditional Processing Method involved in the Process
After each of the fermentation days, the palm fruits were threshed out manually from the bunch. One portion of the 2 kg was boiled for 1 hr while the other was processed without boiling. After boiling, it was allowed to cool after which it was pounded with mortar and pestle.
The mashed palm fruits were transferred into the big plastic bowel after which cold water was added in the other to allow easy skimming of the crude palm oil from the mashed fruit. The extracted oil was subjected to further heating in other to remove moisture. Then the oil was scooped out and then placed for second boiling, after which the oil was filtered and allowed to cool. A measuring cylinder was used to determine the yield of the oil. The other un-boiled portion of the palm fruit equally underwent the same processes, but the fruits were not boiled before pounding. The cooled, measured palm oil was then transferred to a ½ liter dark plastic container and kept away from heat and direct sunlight to avoid oxidative rancidity. The next day, palm oil produced was analyzed for the physicochemical properties.
About 25 ml of diethyl ether with 25 ml of ethanol was mixed and 1 ml of phenolphthalein solution (1%) added and 0.5 g of the oil sample was accurately measured into a clean dry conical flask of known weight and it was dissolved in the solvent. It was titrated with aqueous 0.1 M KOH solutions, shaking constantly until it reaches a pink color, which will persist for 15 seconds is obtained (AOAC, 2002).
Free Fatty Acid Calculation:

Where N is the Normality of NaOH
About 0.5 g of the oil sample was weighed accurately into a clean dry conical flask. 1 g powdered potassium iodide, 20 ml of solvent mixture (2 vol. glacial acetic acid, and 1 vol. chloroform) was added. This was placed in a boiling water bath for 60 seconds. The content was quickly poured into a flask containing 20 ml potassium iodide solution (5%). The flask was washed twice with 25 ml of water. The mixture was then titrated with 0.002 m sodium thiosulphate solution using starch as an indicator (a ml) and the end point was noted. At the same time, the blank test was also carried out by titrating the mixture excluding oil sample (b ml) (AOAC, 2002).
Peroxide Value Calculation:
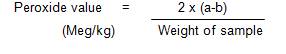
About 0.5 g of the oil sample was weighed into a 250 ml glass stopped bottle and 15 ml of carbon tetrachloride was added to the oil. This was dissolved with 5 ml of Wijis' solution after which the stopper that has been moistened with potassium iodide solution inserted. This was mixed and allowed to stand in a dark cupboard for 30 minutes. After 30 minutes, 20 ml of potassium iodide solution and 150 ml of distilled water were added to the mixture and shaked well. After that, the mixture was then titrated with standard 0.1 M sodium thiosulphate solution using starch as an indication (a ml). A blank test was also carried out, commencing with 15 ml of carbon tetrachloride excluding the oil (b ml) (AOAC, 2002).
Iodine Value Calculation:
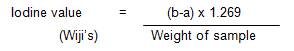
About 1 g of the oil sample was weighed into a dry clean conical flask and 25 ml of 0.5 M alcoholic KOH solution is added to it. The flask was attached to a reflux condenser and heated on boiling water for 1 hour with occasional shaking. At the end of the 1 hour, it was observed that there is an absence of an oily surface. The solution indicated a complete saponification of the oil. 1 ml of phenolphthalein solution (10%) was then added to the mixture while hot and it was titrated with 0.5 M standard hydrochloric acid (a ml). A blank test was also carried out (b ml). The saponification value was calculated from the difference between the blank and sample titration (AOAC, 2002).
Saponification Value Calculation:
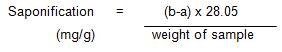
Where b = titre value of blank,
a = titre value of sample, and
28.05 mg of KOH equivalent to 1ml of 0.5 M HCl.
The method described by Onwuka (2005) was employed and it was done using Abbe refractometer.
1 g of the oil sample was frozen. A smear of the frozen oil was made on the heating plate of Fisher-John melting apparatus and covered with an observation lens, and then the apparatus was switched on. The temperatures at which the oil began to melt and when the smear completely melts were observed through an inserted thermometer (Onwuka, 2005).
An evaporating dish was washed and dried in an oven. About 10 ml of oil sample was poured into the evaporating dish and a thermometer suspending at the centre of the dish ensuring that the bulb just dipped inside the oil without touching the bottom of the dish. The dish was heated on an electric stove and gradually, the temperature was noted when it rises. The temperature at which the oil sample gives off a thin bluish smoke continuously was noted as the smoke point. The temperature at which the oil started to flash (when flame is applied) without supporting combustion was recorded as the fire point.
50 ml specific gravity bottle was properly washed with detergent, dried and weighed. The bottle was filled with water and weighed using mettle weighing balance. The bottle was emptied and dried after which it was filled with the oil sample and re weighed (Onwuka, 2005).
Calculation of Specific Gravity:
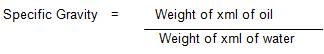
Moisture Content was determined by hot oven method as described by (NAFDAC, 1982). Plastic dish was thoroughly washed and dried in the oven after which it was cooled in the desicator. The weight of the plastic dish was recorded and taken as W1 . About 5 g of the sample was weighed into the previously weighed dish which will then be recorded as W2 . This was placed in a thermostatic well- ventilated oven maintained at 105 oC for 3 hours. At the end of the time, the dish was quickly transferred into a desicator where it was allowed to cool. The weight of thedish and sample was recorded as W3 . Drying and cooling was repeated at 1 hour interval until a constant weight is obtained.
The percentage moisture content was calculated as described in the equation below.
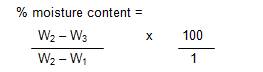
Where,
W1 = weight of empty plastic dish,
W2 = weight of plastic dish and undried sample, and
W3 = weight of plastic dish and dried sample.
Four varied fermentation days: three days, six days, twelve days, and fourteen days for both the boiled and un-boiled samples were identified in this study. The physiocochemical properties of the oil samples produced through the varied fermentation days were presented in Tables 1 and 2.
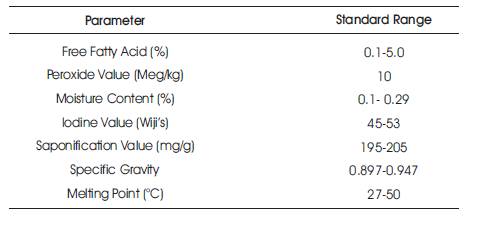
The appendix section shows the Standard Organization of Nigeria (SON) (2000) and Nigerian Industrial Standards (NIS) (1982) standards for the physicochemical properties of palm oil.
The moisture content is an index of its water activity (aw) (Fraziar and Westoff 1978).Prolonged fermentation days increases the moisture content of palm oil which makes the oil to be unstable and prone to microbial attack, thus increasing the FFA content of the oil (Tagoe, et al. 2012). As the days of fermentation increases, the readily degradable particles dissolve in the oil during processing to raise the moisture content in gradual formation of sludge. The slightly high moisture content obtained in this study (Figure 2) may be associated with the heating duration of the palm fruit and the quantity of water added during boiling processes for the boiled samples. There is a correlation significant difference (P<0.05) between the boiled and unboiled samples on the Moisture Content (MC) of the palm oil (Table 1).
The results obtained in this work show that FFA of palm oil increases with the increase in fermentation time for both boiled and unboiled palm fruits (Figure 2). The presence of free fatty acids in palm oil is an indication of the impairment of oil quality. Generally, Free Fatty Acid (FFA) shows the level of rancidity taking place in the oil. The longer the harvested fruits were allowed to stay before they were processed, the higher the FFA of the oil extracted from such fruits. Ripe palm oil fruits contain autolipolytic enzyme which starts to split the fruit oil to fatty acid and glycerol once the fruit is bruised (Bek-Nielsen, 1977; Esechie, 1978; Hartley, 1988). Another reason for the continuous increase in the FFA level, especially after the first two days of fermentation is a result of the microbial decomposition of the black outer pigment of the fruits (Ituem and Modo 2000).
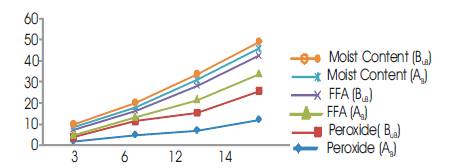
Figure 2. A Graph of Moisture Content, Free Fatty Acid and Peroxide Value Against Fermentation Days
Table 1. Variations of Physical Properties with Fermentation Time And Process Parameters
It was however not surprising that, the values of FFA as high as 8.86% and 8.19% for boiled and unbolied samples respectively were obtained from oil samples after the 14 days fermentation. The free fatty acid values were significantly different (P< 0.05) (Table 2) for both boiled and unboiled samples therefore signifying that, fermentation has a significant effect on the level of rancidity taking place in the oil.
The proxide value of palm oil increased with the increase in fermentation time as shown in Table 2. The values obtained on 3rd day (1.6; 2), 6th day (4.8; 6.4), and 12th day (6.8; 8.4) of fermentation (Table 2) met the recommended (SON, 2000) maximum peroxide value of 10 meq O2 /kg palm oil. The high values of peroxide obtained at the 14th day (12.0; 13.6) of fermentation (Table 2) could indicate the onset of primary oxidation due to lipid degrading enzymes like peroxidase and lipoxygenase. Oxidation, especially the formation of peroxides has been noted to be entirely a man-made feature of palm oil often resulting from processing methods by which hot oil absorbs oxygen from the atmosphere and the process is catalyzed by the presence of certain metals (e.g. iron) and steam (Wolves, 1969; Chi and Tan, 1977).
Peroxidation also makes the oil dangerous for human health when the oil is allowed for longer period of fermentation, as the free radicals generated by this process are proven to be carcinogenic (Rossel, 1999). There is no significant difference (P>0.05) between theboiled and unboiled samples (Table 2).
The atmospheric oxygen and light do not significantly affect the refractive index and the parameter is less useful in the determination of the rate of deterioration of oils. It was observed that, there was a slight decrease in the refractive indices of the palm oil samples with increasing in fermentation days (Figure 3). There is a correlation significant difference (P<0.05) between the boiled and unboiled samples (Table 1).
The specific gravity was seen to increase slightly with increasing fermentation days (Figure 4) and it is reported to increase with increase in the degree of unsaturation that is, with increase in Iodine value (Poku, 2002). This work also observed the increase in Iodine value with increase in fermentation time.
The Iodine value of the palm oil was found to increase with increase in fermentation days of palm fruits (Figure 5). The iodine value is the measure of the level of unsaturation in the oil samples. The value is a useful index of detecting adulteration of oils. It is a measure of the level of unsaturation in oils. The higher the degree of unsaturationin an oil sample, the higher the iodine value of that oil. It therefore follows that the longer the palm fruits were allowed to ferment, the greater the degree of unsaturation in oil sample, which will raise the iodine value of the oil..
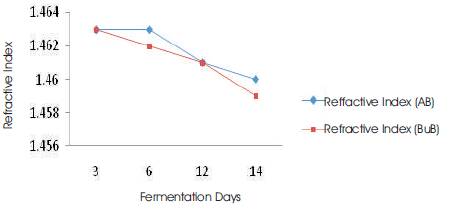
Figure 3. Refractive Index vs Fermentation Days
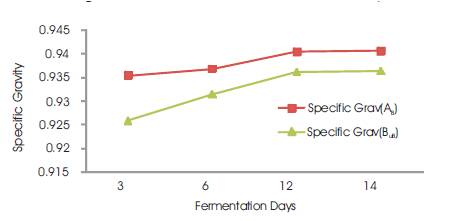
Figure 4. Specific Gravity vs Fermentation Days
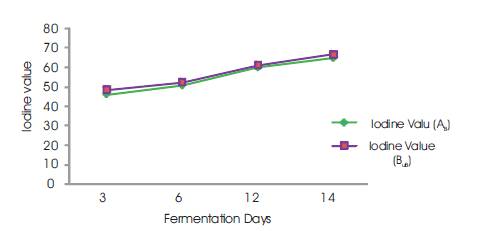
Figure 5. Iodine Value vs Fermentation Days
The iodine values obtained on 3rd (45.94; 48.48) and 6th (50.76; 52.54) days of fermentation (Table 2) were within the standard range of 45 – 53 Wij's as recommended by SON (2002), and NIS (1992) while the 12th (59.89; 61.17) and 14th(64.97; 66.75) day values (Table 2) were above the range. However, these high values show some level of rancidity and deterioration. The results also suggest high level of unsaturation and susceptible to oxidative rancidity. There is a significant difference (P<0.05) (Table 2) between the iodine value of the boiled oil and unboiled oil samples, therefore signifying that fermentation exert a significant effect on the degree of unsaturation of the oil or the iodine value.
The freezing points ranged from 22-28 °C (Table 1). The melting points are below the 27-50 °C as recommended by (SON, 2000). Thus these palm oil samples will remain as liquid at room temperature. The freezing point variation were of significant difference (ρ<0.05) (Table 1). Figure 6 shows a graph of Smoke Point and Flash Point against Fermentation Days.
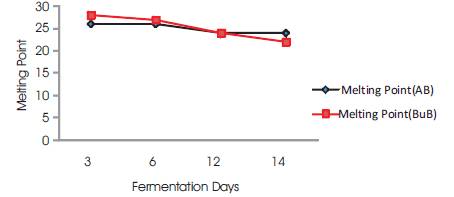
Figure 6. Melting Point Vs Fermentation Days
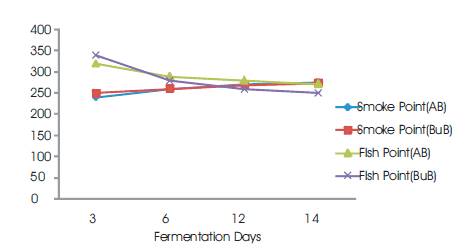
Figure 7. Smoke Point and Flash Point vs Fermentation Days
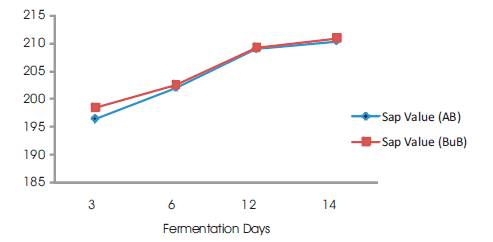
Figure 8. Saponification Value vs Fermentation Days
The smoke value ranges between 240-275 °C. The correlation coefficient showed a significant difference (ρ<0.05) (Table 1). This high value is an indication of suitability of this palm oil for frying purposes
The flash point of palm oil was found to decrease with increase in fermentation time (Figure 7). This is true for both boiled and unboiled palm fruits. The higher the flash points of oil, the lesser the susceptibility of the oil to catch fire during frying. Based on this, prolonged fermentation such as more than 6 days is not recommended.
The results obtained in this work show that, the saponification value increases with increase in fermentation time (Figure 8). Saponification Value (SV) is an indication of the molecular weights of triglycerides of oils. High saponification value indicates high proportion of low fatty acids since saponification value is inversely proportional to the average molecular weight or length of fatty acids.
The saponification values were found to increase with increase in fermentation days for both samples. This trend explains that, with the long storage of this oil, smaller fatty acid molecules are likely to be formed which increase the SV. The values obtained were between 196.35-202.52 mg KOH/g for 3 and 6 days of fermentation. These values are within the recommended range of 195-205 mg KOH/g for palm oil (SON, 2000; NIS, 1992). These values are indication that the oils are well suited for soap making.
The experimental investigation on the effect of fermentation on the physicochemical properties of palm oil led to the following conclusions:
Based on the experimental results, boiled palm fruits resulted in lower Free Fatty Acid, Peroxide value, Iodine value and Saponification value which further suggests that the process of boiling fermented fruits should be encouraged. As these chemical properties; Free Fatty Acid, Peroxide value, Iodine value and Saponification value all increased with fermentation time, fermentation of palm fruits prior to palm oil production is not recommended.
Standard Organization of Nigeria SON (2000) and Nigerian Industrial Standards NIS (1982) standards for the physicochemical properties of palm oil