Figure 1. SEM Cross-sectional Micrographs: (a) After Thermal Cycling at 1373 K for 30 hours and (b) After Thermal Cycling at 1373 K (Zhang et al., 2003)
This paper discusses the effects of the pre-coating treatment and the bond-coat composition on the lifetime and the behavior of thermal barrier coating (TBC) systems. Some literature based analyses have been made on the influence of pretreating a thermal barrier coating composed of a single crystalline Ni based super alloy CMSX-4, platinum aluminide bond coat and an Beam Physical Vapor Deposition (EB-PVD) processed ceramic top coat, prior to deposition of the ceramic top coat. Some analyses show that the specimens annealed in the oxygen containing ArH atmosphere for 4 hours at 1080 0C have a higher cyclic lifetime as compared to the standard treated specimens. The increase in lifetime can be attributed to the formation of a stable and defined thermally grown oxide prior to and during TBC deposition. Also, the bond coat with the silicon gradient effectively prevents the diffusion of Mo thereby increasing the TBC lifetime.
Thermal barrier coatings (TBCs) are advanced materials which are deposited on metallic surfaces operating at elevated temperatures in order to protect the metal substrate from the aggressive environment. Their prime function is to thermally insulate the metal components. Greater fuel efficiency can be achieved at high temperatures which expose the components to extreme service conditions. TBCs are designed to improve the thermal efficiency of components without increasing the surface temperature of the substrate alloy enabling the components to operate at temperatures above the melting point of the alloy. The thermal conductivity of the TBCs dictates the temperature difference across the coating and the heat loss or gain. TBCs are usually made of refractory oxide ceramics. Zirconia oxide has emerged as one of the most preferred materials for TBCs. The major driving force for the development of TBCs has been the benefits to be gained from the extended life of metallic components in the extreme temperature conditions. TBCs have found a great number of high temperature applications such as the aviation industry, jet engines, turbine blades, etc. A number of studies have been carried out in order to increase the lifetime of TBCs. This review focuses on effects of the pre-coating heat treatment and the bond coat composition on the lifetime and the thermal behavior of TBC systems.
It is a well-known that an increase in the operating temperatures of turbines would increase their efficiency. Turbine blades are usually manufactured with a basic service temperature range of 960-1100 °C and a peak service range of 1065-1300 °C. The operating temperatures may reach an excess of 1500 °C in commercial and military aircrafts whereas the maximum operating temperatures of nickel based super alloys used in turbine blades production ranges from 850-1100 °C. Relentless increase in operating temperatures have been achieved through the improved alloy design, the development of blades composed of textured microstructures and subsequently single crystals, and internal cooling by air flow through internal channels cast into the component. More recent increases in operating temperatures have been enabled by deposition of TBCs on high-temperature gas turbine components. TBC systems comprise of thermally insulating materials which can enable a significant temperature difference between the load bearing alloy and the coating surface. The lowering of the temperature of the substrate alloy prolongs the lifetime of the turbines by safeguarding against the extremely aggressive thermomechanical environment, creep and thermal fatigue ( Baufeld & Schulz, 2006; Evans et al., 2001; Zhang et al., 2003).
Recent TBC systems consist of the following components.
The ceramic top coat is a thermally insulating oxide. Zirconia oxide (ZrO2) has emerged as a preferred option for these purposes. The structure of ZrO2 changes from the monoclinic to the tetragonal when heated above 1000 0C. The accompanying volume changes can result in severe spalling of the ceramic layer which is detrimental for the system. The ZrO2 is stabilized by the addition of yttria to form yttria stabilized zirconia which has a low thermal 2 conductivity (~1 W/m2 K). The ceramic coating is deposited by using an electron beam physical vapor deposition (EBPVD) technique which results in a columnar microstructure. This type of the structure exhibits both the high stress and the thermal shock tolerance. The individual columns in this columnar microstructure also prevent the buildup of any tensile stresses and match the coefficient of thermal expansion (CTE) differences between the thermal barrier coating and the base metal ( Evans et al., 2001).
The thermally grown oxide (TGO) which develops at the interface between the TBC and the bond coat at elevated temperatures determines the lifetime of the TBC and its ultimate failure mode. The thermally grown oxide is usually aluminum oxide. The bond-coat is an aluminum-rich composition, so it promotes a formation of the highly stable oxide as a protective layer. Alumina is most preferred oxide due to its low diffusivity and the superior adherence property. The formation of the TGO can occur initially prior to and during the EB-PVD process of the zirconia coating as there may be some oxygen in the deposition chambers. The rate of aluminum oxide formation depends on the activity of oxygen as well as time and temperature. The thickness of the TGO layer is generally much less than that of the TBC, typically only a few microns. However, it is dense and does not comply as the TBC. Consequently, the stresses in the TGO typically range between 3 and 6 GPa. The thermal expansion mismatches with the bond coat and significantly contribute to the failure of the TBC system. Some studies showed that a failure in EB-PVD TBCs is mostly initiated at or near the TGO, along the TGO/bond coat interface ( Evans et al., 2001).
The bond coat is arguably the most crucial component of the TBC system as its chemistry and microstructure influences the nature of the TGO. Bond coat system consist of two major components:
The NiCoCrAlY bond coats usually contain two phases:
The γ/γ’ phases contain other elements in the solution. The yttria is added to enhance the adhesion of the TGO by gettering the sulphur which diffuses from the superalloy. The Pt-aluminide bond coats are usually made up of a single β phase with platinum in the solid solution. The diffusion of aluminium into the superalloy results in the formation of the γ’ phase at the β grain boundaries. The interface between the TGO and bond coat is also of the critical importance as it can be embrittled by segregation of sulphur from the substrate but this is usually prevented by dopant elements in the bond coat such as yttrium.
Some studies evaluate the ways of increasing the lifetime and the thermal cyclic behavior of TBCs by pretreating the platinum aluminide bond coat prior to the ceramic top coat deposition.
Zhang et al., (2003) conducted the tests on samples of Ni3Al based alloy IC6 containing 14% molybdenum, 7.8% aluminum, 0.04% boron and 0.1% carbon. This alloy has been developed for turbine blades and vanes of advanced jet engines and other high temperature structural components. Two different bond coats (MCrAlY, Ni-24, Co-15, Cr-8, Al-1wt% Y and MCrAlY) of about 50 μm thick were deposited on 15 mm x 10 mm x 3 mm rectangular alloy samples by EB-PVD process. Isothermal oxidation of the samples has been carried out in air at 1373 K for 30 minutes followed by forced air cooling up to the room temperature in 5 minutes. The weight gain of these samples has been measured using a TG328 analytical balance. SEM and EDS microscopy were used to analyse the microstructure and composition distribution of the samples after an oxidation.
Another set of tests has been carried out by Baufeld and Schulz (2006), on a single crystalline Ni-based super alloy CMSX 4. The bond coat has been prepared by electroplating platinum and diffusing aluminum by high activity chemical vapor deposition onto 65 mm x 6 mm x 6 mm cylindrical rods of CMSX-4. An YSZ top coat has been applied using EB-PVD process at 1000 oC with a controlled amount of the oxygen. Some of the bond coated samples were lightly grit blasted using alumina grit followed by annealing at 1080 0C in a vacuum with an oxygen partial pressure of ~10-5 m bar or ArH atmosphere, at 10 l/h ArH flow, 70 m bar pressure and ~6 x 10-3 oxygen partial pressure for 4 hours. The samples were lightly grit blasted before depositing the TBC. The non-heat treated samples were grit blasted with 220 mesh alumina at 20 psi pressure. Thermal fatigue cycling tests were performed until failure on the non-heat treated, vacuum annealed and ArH annealed samples. Two samples from each category were treated at a peak temperature of 1100 °C for 1 hour. Heating time has been set at 6 minutes and total cooling time up to the room temperature has been 10 minutes. The phases at the surfaces of the coated samples and annealed samples were analysed with Siemens D5000 XRD. SEM microscopy has been carried out to determine the microstructure of selected samples. EDX element analysis has been also conducted to analyze the compositions of the test samples ( Chai, 2017; Lin Chen & Yueming, 2018; Lin & Li, 2016).
Cross sections of SEM images for the IC6 alloy coated with MCrAlY revealed a presense of the interdiffusion zone between the substrate and the bond coat layer even at the deposited state. A brittle phase with cavities resulting from the diffusion of Mo, Ni and Si elements has also been observed ( Zhang et al., 2003). After isothermal oxidation the thickness of the interdiffusion zone increases. However, the brittle phase, observed initially, is disappeared. It is shown that Mo diffuses into the bond coat layer resulting in high Mo content at the surface of the bond coat as indicated in Tables 1 and 2. This would attribute to the spallation of the ceramic top coating. A thick TGO layer is also formed at the interface between YSZ and the bond coat layer after thermal cycling as show in Figure 1 (a) and (b) ( Zhang et al., 2003). It would result in failure of the TBC. Contrary to these observations, only small amounts of Mo were detected in the samples with McrAlYSi bond coat as indicated in Tables 3, 4 and 5. It proves that the addition of the silicon element supresses the diffusion of Mo into the bond coat layer and therefore, it increases the TBC lifetime ( Zhang et al., 2003).
Figure 1. SEM Cross-sectional Micrographs: (a) After Thermal Cycling at 1373 K for 30 hours and (b) After Thermal Cycling at 1373 K (Zhang et al., 2003)
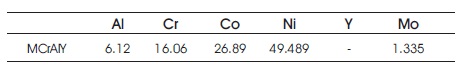
Table 1. Composition of As Deposited Bond Coat (wt %) (Zhang et al., 2003)
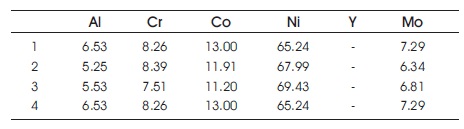
Table 2. Composition Distribution after Isothermal Oxidation without Silicon Addition for 60 hours (Zhang et al., 2003)
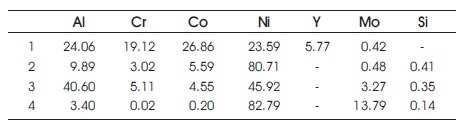
Table 3. Composition Distribution after Oxidation with Silicon Addition for 100 hours (Zhang et al., 2003)
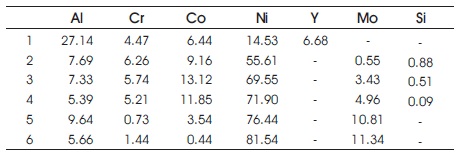
Table 4. Composition Distribution after Oxidation with Silicon Addition for 30 hours (Zhang et al., 2003)
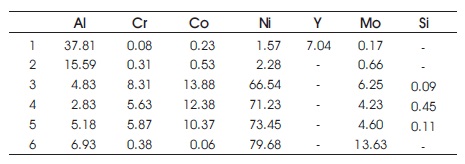
Table 5. Composition Distribution after Oxidation with Silicon Addition for 340 hours (Zhang et al., 2003)
Baufeld and Schulz (2006), demonstrated that the bond coat in the As-coated samples is 130 μm thick and it is made up of two layers. This was in contrast to the results obtained in Zhang et al. (2003). However, the phases present in the bond coat depend on the process conditions employed during the bond coat manufacturing process. The inner layer which is closer to the substrate consists of a β NiAl phase containing Co, Ni and some precipitates. An outer layer comprising of PtAl phase with 2 Ni, Cr, Ti and other elements and a β NiAl phase containing Co, Pt and Cr elements. The concentration of Al in the bond coat increases towards the surface whereas the Ni concentration decreases. Platinum has been in the outer layer only. The concentration of elements which diffused from the substrate and the precipitates decreases towards the surface. The precipitates contain W, Re, Ta and Mo which diffused from the substrate during the CVD process. Oxides are found in the outer layer because they were formed when the samples were grit blasted prior to the bond coat manufacture process. After annealing (ArH and vacuum annealing), similar compositional profiles were observed. Figure 2 (a) and (c) shows the cross sections of ArH annealed and Figure 2 (b) and (d) shows the vacuum annealed specimens before and after TBC deposition. The outer layer remains two-phased and the inner layer remains single-phased with some precipitates. A slight decrease in the Al concentration and an increase in the Ni concentration were observed. A thin TGO layer has been found on the surface of the ArH annealed samples. A 5 μm thick porous phase of NiAl which is depleted of Pt has been found on the surface of the vacuum annealed samples as in Figure 2 (b). After TBC deposition no significant compositional differences were observed since the TBC deposition temperature has been lower than the heat treatment temperature. However, due to the presence of some oxygen during TBC deposition, a thin TGO layer ~5 μm has been formed on all samples at the TBC and bond coat interface as Figures 2 (c) and (d). The pore walls of the vacuum anealed samples were covered by TGO. Similar compositional profiles were observed for all three cases. A significant increase in the concentration of Ni and a decrease in the concentration of Al has been observed in the bond coat. After thermal cycling the composition has been found to be similar in all samples. There has been no evidence for any martensitic transformation as predicted by theories. TGO microstructure has been slightly different in the samples. In the observed failure modes there has been no extensive rumpling of the TGO as indicated in Baufeld and Schulz (2006).
Figure 2. (a) and (c) Cross Sections of ArH Annealed Specimens, (b) and (d) Cross Sections of Vacuum Annealed Specimens Before and After TBC Deposition (Baufeld & Schulz, 2006)
The weight gain has been very low for the samples with TBC coating. It increased for those with MCrAlYSi and MCrAlY bond coats. The weight gain has been highest for the samples which had no coating as shown in Figure 3. The lowest weight gain for the alloy with TBC indicated that the TBC system protects the alloy from the oxidation. The high content of molybdenum in the IC6 alloy favours its oxidation to molybdenum oxides which are volatile at high temperatures. The evaporation of this oxides may result in the formation of point defects in the material. These defects render the bond coat susceptible to further oxidation. The high weight gain is attributed to the formation of oxides at the elevated temperatures. In case of the samples which have TBC evaporation of molybdenum oxides is hindered by the protective top coat resulting in reduced further oxidation and consequently low weight gain. The build up of molybdenum oxides between the bond coat and the ceramic coating lowers the thermal cyclic lifetime of the samples compared to other nickel based alloy substrate TBC systems ( Zhang et al., 2003).
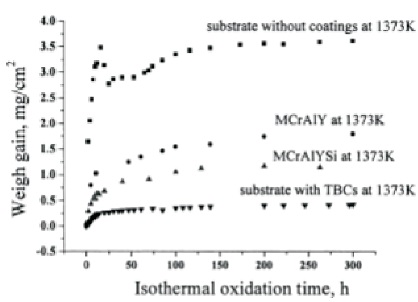
Figure 3. Isothermal Oxidation Curves (Baufeld & Schulz, 2006)
The thermal fatigue lifetime depends significantly on the treatment prior to the TBC deposition. As compared to the standard treatment (grit blasting only), the cyclic lifetime has been increased by a factor of 2 and 3 for vacuum annealed and ArH annealed samples, respectively as shown in Figure 4. However, the lifetime of samples with standard pretreatment is lower than what has been reported by Gell et al. (2004). The standard grit blasting operation does not provide a well-defined TGO prior to the deposition. Hence it results in the low cyclic lifetime. A dense alumina phase is developed after TBC deposition because of the low temperatures used in TBC deposition and the fast TGO growth is attributed to the formation of the alumina which is formed at low temperatures. The formation of pores on the bond coat surface during the annealing phase is attributed to the evaporation of Al as a result of the low vapor pressure of aluminum. Platinum is also volatile at high temperatures. Therefore, the platinum aluminide phase has been lost. The pores result in an increased TGO growth during the vacuum annealing due to the increased surface area exposed to the oxygen. The large pores were not completely filled during TBC deposition probably because the pores are too small for the condensing vapor to penetrate them or because of the differences in the thermal expansion between the substrate and the TBC. The porous surface hinders the formation of a perfect bond coat and TBC interface. Hence, the low cyclic lifetime as compared to ArH annealed samples. A higher oxygen partial pressure during ArH annealing hinders the evaporation of the platinum aluminide phase. Thus the relatively smooth surface will be conserved and a tight interface with the TBC will be achieved. Although some γ-alumina TGO with poor adherence may be formed due to the low temperatures used in TBC deposition, this phase is transformed to the more adhering α-alumina phase as a result of the high temperatures preceding TBC deposition. This results in a high cyclic life for ArH annealed samples ( Evans & Hutchinson, 2007; Limarga, 2011; Scardi, 1995).
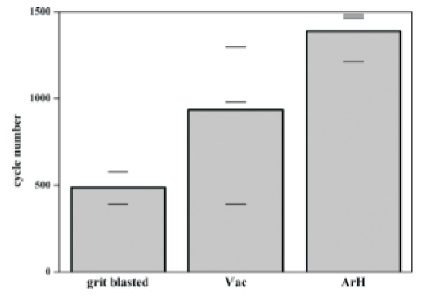
Figure 4. TBC Lifetime for the Different Pre-treatments (Baufeld & Schulz, 2006)
The use of the gradient composition MCrAlY-Si bond coat inhibits the diffusion of Molybdenum in Ni3Al based alloy IC6 TBC systems which in turn increases their lifetime ( Zhang et al., 2003). Annealing proceeding TBC deposition improves the TBC lifetime in Ni-based CMSX-4 TBC systems. ArH annealing increases the TBC lifetime by a factor of almost three. This improvement is attributed to the defined formation of a TGO layer prior to and during TBC deposition. The formation of pores during vacuum annealing adversely affect the strength of the TBC/BC interface thus lowering the TBC lifetime. However, the role played by hydrogen in the ArH annealing is not fully understood, thus further study is recommended in order to fully explain its influence.