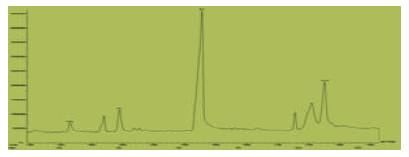
Figure 1. GC/MS spectrum of Soyabean methyl ester
The main objective of this study is to experimentally investigate the use of soyabean oil methyl ester and its blends in a single cylinder direct injection compression ignition engine. The fatty acid methyl esters in soyabean methyl ester were found to be Myristic acid, Palmitic acid and Stearic acid in prominent proportions. The blending ratios of 5%, 10%, 15% and 20% of SOME with diesel were used in this study with varying loads. The combustion study such as in-cylinder, maximum peak pressure, rate of heat release and ignition delay are analyzed and found that SOME 20% blend exhibits maximum cylinder pressure and better heat release characteristics. The effect of injection timing is analyzed by advancing and retarding the injection timing by 5oCAD and found to have positive effect on combustion efficiency with advancement of injection timing
The mono-alkyl esters of the oils and fats from vegetable and animals are said to be biodiesel. The biodiesel st gained more importance at the start of 21st century because it was accepted widely at the end of 20th century that conventional sources of petroleum derivatives are soon depleting and a suitable replacement has to be achieved before the crises period. The main content of vegetable oil and animal fat are long chain fatty acids called triacylglycerols (TAGs) [2][4]. These fatty acids are transformed into fatty acid methyl or ethyl esters through transesterification process in which a alcohol mainly methanol reacts with triacylglycerols in the presence of a sodium hydroxide or potassium hydroxide as a catalyst to form one molecule of FAME liberating mono acyl-gylcerol in the presence of heat. Biodiesel is very similar to petrodiesel in most comparisons. It can be blended with diesel in various blend ratios and even can be used directly as straight vegetable oil in compression ignition engine in B100 with slight modification in the engine. Biodiesel also contains oxygen which enhances its self lubricating properties than diesel. The heating value of biodiesel is lower than 12% to diesel because of increased oxygen content[7][12]
Now-a-days, biodiesel are produced through various methods includes thermal cracking, pyrolysis, microemulsion and transesterification, in which transesterification is the most common, successful and economical for the production of biodiesel[1]. Several researchers have experimentally investigated the use of rice bran oil and soyabean oil in compression ignition engine as a suitable replacement of diesel fuel. They have suggested that the optimal transesterification condition for rice bran oil and soyabean oil was found to be 55oC-60oC, 1 hr 20 min reaction time, and 8:1 molar ratio to methanol with 0.72%-1% of catalyst. The soyabean oil contains high free fatty acid which requires several additional steps of chemical reaction along with transesterification to transform them into biodiesel. This FFA reduces the transesterification efficiency, yield of FAME's and increases the production cost of biodiesel[13]. The main disadvantage in most studies is that, they treat the combustion of biodiesel in same way as diesel fuel without considering the chemical structure, chemical kinetics, atomic arrangement and oxygen content. These variations have a deep impact in combustion and performance as they are oxygenates and also results in less particulate formation due to oxidative properties.
In this study, blending of biodiesel with commercial diesel in 5%, 10%, 15% and 20% ratios, engine load, speed, power, compression ratio, in cylinder pressure rise, injection pressure and timing and ignition delay were considered and compared with standard diesel.
Soyabean oil seed production is estimated to be 123.2 tones all over the world and 4.3 tones in India per year. In India, 0.63 tones of Soyabean oil are used for various purposes [17][18]. Soyabean oil is obtained by drying, crushing and grinding the oil seed in a standard device. A two step acid and one step base catalyzed transesterification process is carried out to produce SOME. In the first step, 1% of concentrated sulphuric acid is added to the soyabean oil to neutralize and reduce the free fatty acid content from 8% to 1.25%. The solution is o heated to 50oC and kept in a rotating agitator at 500 rpm allowing a reaction time for 1 hr. The second step was carried out using sodium hydroxide and methanol to form sodium methoxide. Different molar ratios were investigated including 0.1, 0.2, 0.3, 0.4, 0.5 and 0.6 to analyze the effect of sodium hydroxide on soyabean oil[1][16]
Further the base catalyzed transesterification process was carried out using sodium hydroxide. An investigation has been carried by pouring methanol-soyabean oil in o various test tubes maintaining at 60 C and NaOH was added to every test tube by 0.5%, 0.75%, 1%, 1.25%, 1.5%, 1.75% and 2.0% by weight to analyze the formation of SOME and glycerol. It was found that 1.5% of NaOH yielded better SOME while 2% and above negative improvement by soap formation. The solution was 0 maintained at 60oC and placed in an agitator at 500 rpm with 2 hrs reaction time. After 24 hrs of separation period, the SOME was separated from glycerol and washed with 5% of distilled water. The FAME's were identified using GC/MS as given in Table 1[14](Figure 1)

Figure 1. GC/MS spectrum of Soyabean methyl ester
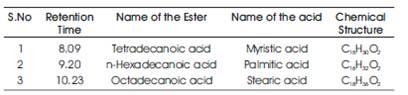
Table 1. Fatty acid methyl esters in Soyabean biodiesel
A single cylinder naturally aspirated direct injection compression ignition engine was used for this study with the specifications given in Table 2
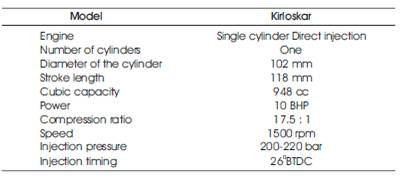
Table 2. Specification of Test Engine
The test engine was coupled with an eddy current dynamometer providing a maximum power of 10 BHP with 0.1% uncertainty. The engine was operated at different torque and different speeds to analyze the efficiency. The engine speed was measured using inductive speed pick up and the in-cylinder pressure was measured using Kistler 5645A model pressure pick up with a crank angle calculator for amplifying the pressure signal. The static injection timing was varied by spill method. A semicircular protractor is placed towards the flywheel of the engine which designates the TDC and BDC point. The protractor has a resolution of 0.5o and the injection timing is marked with a pointer arrow. There were two leafs of thickness 0.24mm in the fuel injection pump revealing the factory setting timing of 334 CAD (i.e) 26oBTDC. For advancing the injection timing one leaf was removed from the conventional setting to get 50CAD advancement and one leaf was added to retard 50CAD of injection timing. The schematic layout of the experimental setup is given in Figure 2
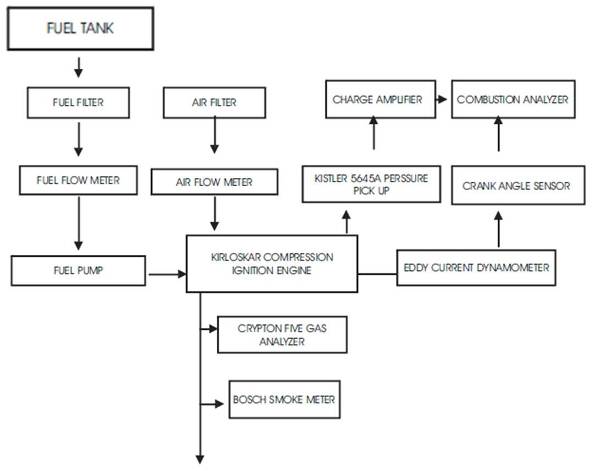
Figure 2. Schematic layout of Experimental setup
The heat release analysis is carried out using In-cylinder pressure rise and the relation is given in equation (1)
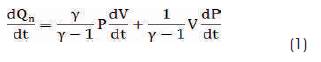
Where γ is net heat release rate, P is instantaneous cylinder pressure and V is instantaneous cylinder volume. The temperature of cylinder wall is assumed to be 725 K. The In-cylinder pressure data was acquired at every 0.36o CA upto 200 cycles and used for calculating the heat release rate. In addition to the above, the assumptions like homogenous air fuel mixture, uniform pressure and temperature are made throughout the combustion process [11]
Figure 3 shows the variations of in-cylinder pressure for diesel fuel at advanced, rated and retarded injection timing. The fuel is injected into the intake manifold at 15oCAD before top dead center conventionally[3]. The fuel shows an increase of in-cylinder pressure upto 63 bar at 370 CAD during advanced injection timing while rated and retarded injection timing shows a gradual decrease in maximum in-cylinder pressure by 4%-6% (i.e) rated injection timing exhibits 60 bars and retarded timing shows 59 bars. This may be due to prolonged time for reaction of combustible mixture of diesel fuel in CO2 and O2 and hence more heat is produced[5][6]. During rated 2 and retarded injection timing, the duration is ultimately short and fuel conversation period is relatively shorter than advanced injection timing. Figures 4 to 7 show the variation of peak cylinder pressure when soyabean methyl ester blends with diesel is used as a fuel. The SOME is blended with commercial diesel in 5%, 10%, 15% and 20% blend ratios. When the pressure curves are compared with each other, there is a slight variation in the start of combustion, for SOME 5% blend as shown in Figure 4. The start of combustion occurs 2 CAD earlier because of oxygen content in SOME and shorter ignition delay. It also shows cylinder pressure for SOME 5% at advanced, normal and retarded injection timing in which advanced injection timing exhibits better increase in peak pressure by 5% to 7%. At retarded injection timing, the cylinder pressure drops to 60 bar which is lower than advanced injection timing due to relatively shorter ignition delay. Figures 5 to 7 show combustion curves in SOME 10%, SOME 15% and SOME 20% bends for advanced, rated and retarded injection timing. The curve ranges almost similar with respect to its injection timing in which SOME 20% blend shows a maximum in-cylinder pressure of 74 bars at advanced injection timing while SOME 15% blend shows 68 bars at same. The retarded injection timing for all blends of SOME shows 7% to 9% decrease in cylinder pressure [9][8][15]
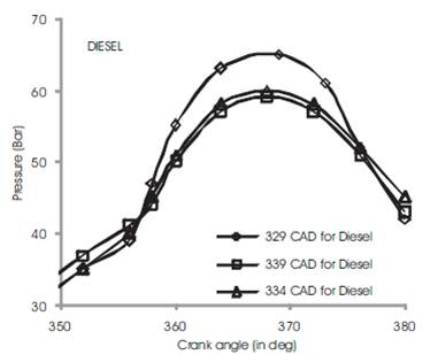
Figure 3. Comparison of cylinder pressure rise for Diesel at various injection timing
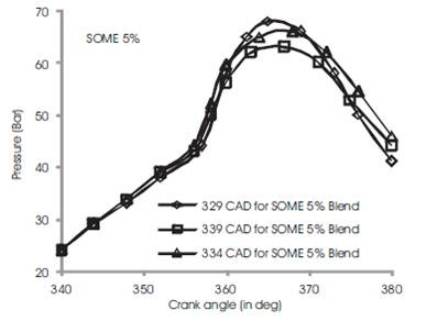
Figure 4. Comparison of cylinder pressure rise for SOME 5% at various injection timing
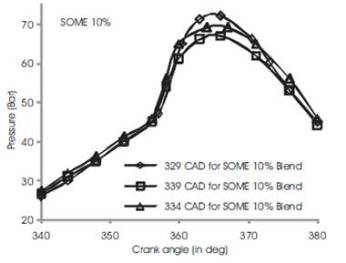
Figure 5. Comparison of cylinder pressure rise for SOME 10% at various injection timing
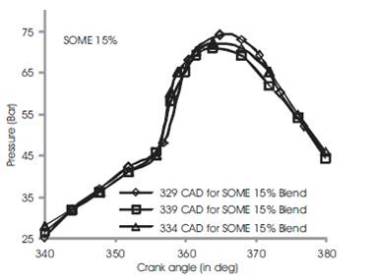
Figure 6. Comparison of cylinder pressure rise for SOME 15% at various injection timing
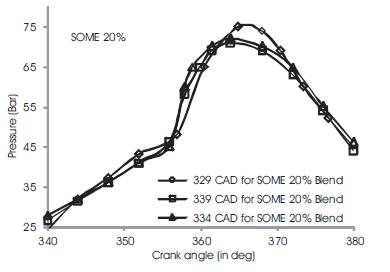
Figure 7. Comparison of cylinder pressure rise for SOME 20% at various injection timing
It can be seen from Figures 8 to 10 which reveal the rate of heat release for diesel, SOME 5% Blend, SOME 10% Blend, SOME 15% Blend and SOME 20% Blend ratio at advanced, rated and retarded injection timing respectively. From the figures it can be noted that diesel fuel blended with soyabean methyl ester have relatively shorter ignition delay than diesel fuel and it increases with a increase in SOME blends which is also discussed under in-cylinder pressure. At 3/4th loading condition, the rate of heat release for soyabean biodiesel is lower than diesel because of longer premixed combustion phase. This may be also due to shorter ignition delay at rated injection timing. But diesel fuel shows higher heat release than SOME because of longer ignition delay [10]
During advanced injection timing as shown in Figure 8, SOME 20% blend shows higher heat release rate at part and 3/4th loading condition. The heat release rate of soyabean biodiesel is lower than diesel because of longer premixed combustion phase. This may be also due to shorter ignition delay at rated injection timing. But diesel fuel shows higher heat release rate than SOME because of longer ignition delay. During advanced injection timing, SOME 20% blend shows higher heat release at part and 3/4th load than diesel which may be due to enhanced premixed combustion period and shorter ignition delay (Figure 9). During this stage more quantity of fuel is admitted into the combustion chamber and total period for combustion is also higher resulting in higher heat release rate. Retarded injection timing for SOME blend and diesel is shown in Figure 10. During this period the heat release rate for diesel is 73 J/CAD which is 10% to 12% higher than all blends of SOME which may be due to shorter ignition delay and time as fuel consumption is less. This may be also due to poor premixed combustion phase due to shorter ignition delay[8]
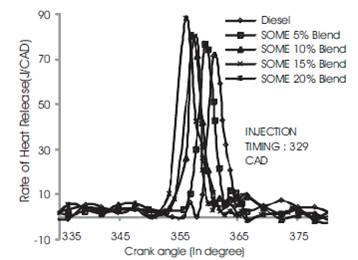
Figure 8. Comparison of Rate of heat release for all Blends at advanced injection timing
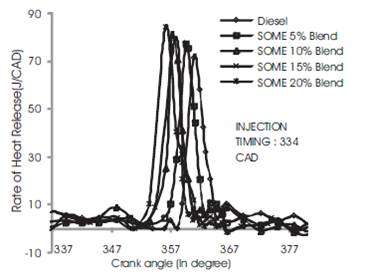
Figure 9. Comparison of Rate of heat release rise for all Blend at standard injection timing
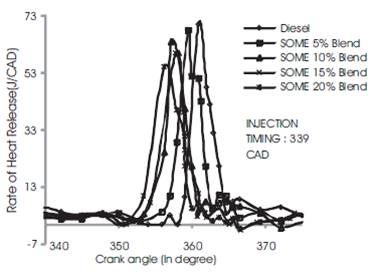
Figure 10. Comparison of Rate of heat release rise for all Blend at retarded injection timing
Soyabean biodiesel was found to be one of the suitable alternative fuels in today's world. The properties of SOME and its blends closely resemble the conventional petrodiesel and therefore minimal or no modifications in the existing engine may be needed. The performance and combustion optimization mainly depends on the feed stock and optimal blending level with diesel. In the present study, SOME and its blends were used to analyze the combustion efficiency in compression ignition engine. It is identified that soyabean methyl ester consists of Myristic acid, Palmitic acid and Stearic acid. It is concluded that soyabean biodiesel results in 6%-7% better in-cylinder pressure than diesel in all loading condition and the ignition delay reduces consistently with addition of SOME with diesel. The rate of heat release also showed 7%-9% increase with 20% SOME blend which is optimum. Advancing the injection timing by 50CAD from the rated injection timing showed better improvement in maximum in-cylinder pressure and heat release rate. As a further extension of this study wear analysis can be performed to assess the performance of the engine on a long run